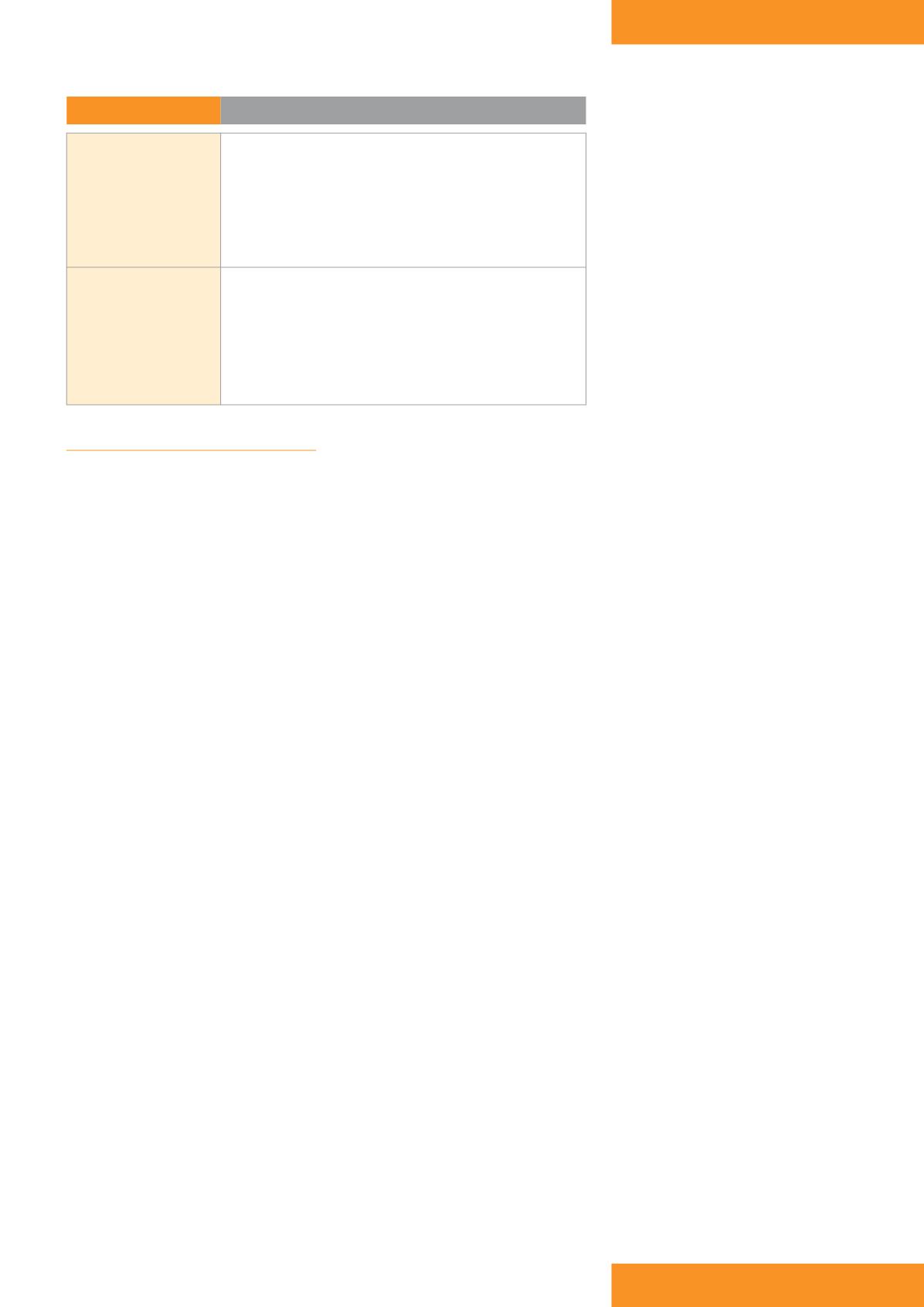
MODE OF ACTION
JCN supplement
2015,Vol 29, No 5
7
MODE OF ACTION
To date, knowledge of the mechanisms
of action of NPWT has largely been
gained from animal and laboratory
studies and there appears to be no
single mechanism responsible for the
clinical benefits seen. NPWT’s mode
of action can be summarised as:
Micro- and macromechanical
deformation of tissue
Changes in blood flow patterns
Removal of fluid and reduction
in oedema
Wound homeostasis (prevention
of desiccation, minimises
contamination).
Tissue deformation
The beneficial effects of NPWT on
wound healing are thought to depend
on the delivery of mechanical forces
(often termed macromechanical and
micromechanical forces) to the tissue.
The notable contraction of
wounds upon application of NPWT
demonstrates the macromechanical
force or tension that is being applied
to the tissue under vacuum. It is
thought that the application of tension
upon the tissue edges‘stretches’the
tissue and stimulates cells to undergo
increased proliferation and matrix
production, resulting in the growth
of new skin tissue (granulation and
epithelialisation) (Saxena et al, 2004).
Interestingly, in studies of tissue
contraction in animals where NPWT
was removed 48–72 hours after
application, wounds did not revert
back to their original size. This
demonstrates a degree of permanency
reduction of between 15–32% in
wound volume per week (Campbell
et al, 2008; Bondokji et al, 2011;
Dorafshar et al, 2012).
Blood flow changes
Despite being one of the most widely
studied effects of NPWT, tissue
perfusion remains a subject of intense
debate. Spanning almost 20 years of
research, experimental studies have
shown that NPWT results in both
an increase and decrease in tissue
perfusion, which very much depends
on the method of detection used
in the study, location and pressure
levels being applied. This area of
research has mostly been limited
to experimental studies with little
clinical evidence owing to the invasive
procedures involved in attempting to
measure local tissue perfusion.
Morykwas et al (1997) were the
first to report changes in blood flow
associated with NPWT. Using laser
Doppler flowmetry in a pig wound
model, it was shown that periwound
blood flow increased upon the
application of NPWT. Subsequent
studies have shown that NPWT
causes an immediate increase in blood
flow in the periwound area (2cm from
the wound edge); whereas blood flow
at the wound edge is reduced, creating
a‘zone of hypoperfusion’(Wackenfors
et al, 2004).
This reduction in blood flow
observed at the wound edge is most
likely due to the compression caused
by the wound filler material pressing
against the surface of the wound. It is
not known whether wounds progress
because of, or despite this zone of
hypoperfusion. One theory is that the
subsequent hypoxic environment is
a potent stimulator for angiogenesis,
which is also a key precursor to
granulation tissue formation (Malsiner
et al, 2013).
Removal of fluid and oedema
Wound exudate, particularly that
seen in chronic wounds, can contain
elevated levels of inhibitory factors
such as proteases and inflammatory
mediators that impair wound healing
and keep the wound in a stalled state
(Schultz et al, 2003). By removing
excess fluid and reducing tissue
oedema, the wound is more likely to
in the contraction effect and supports
the hypothesis that NPWT contributes
to increased cell and matrix
production (Malmsjö et al, 2012).
Perhaps the most notable of all
NPWT’s effects is the stimulation of
tissue granulation and a dramatic
improvement in the appearance
of the wound bed. This relatively
rapid phenomenon is the result of
microscopic interactions between
tissue and wound dressing
materials placed under vacuum.
The combination of both negative
and positive pressures creates
micro-deformation of tissue and the
resultant strain generates increased
responsiveness to growth factors,
cell proliferation, production of
extracellular matrix and angiogenesis
(formation of new blood vessels)
(Wilkes et al, 2009).
Numerous animal studies
have recreated the stimulation of
granulation tissue in open wounds
using a variety of wound fillers and
pressure levels (Morykwas et al,
1997; Malmsjö et al, 2012), while
histological analysis of clinical biopsies
following NPWT clearly shows a more
angiogenic environment (Malsiner et
al, 2013; Fraccalvieri et al, 2014).
It is the combination of these
macro- and microdeformations
(wound contraction and filling of
tissue defects with new granulation
tissue) that ultimately leads to the
visible reduction in wound area
and wound depth. Rate of volume
reduction varies by wound type,
but clinical studies demonstrate a
Table 1:
NPWT benefits
Wound
›
Promotion of granulation tissue
›
Improvement in blood flow
~ increased delivery of oxygen and nutrients
›
Control of exudate
~ decreased wound oedema and congestion
~ improved wound environment
›
Reduced risk of infection
Patient and carer
›
Greater patient comfort:
~ better management of exudate
~ reduced frequency of dressing changes
~ reduced wound odour
~ increased mobilisation
›
Reduction in wound area and depth
›
Reduced overall treatment costs